Thank you for Connecting with Trellus Elevate™
This Reference Guide was designed to ensure you have everything you need to partner with us in delivering the highest quality of resilience-based integrated support to you and your patients with IBD.
Please do not hesitate to contact us via email elevate@trellushealth.com or by phone at 800.989.5503 with any questions or any additional help we can provide for you and your patients.
Thank you for joining us in reimagining resilience for chronic GI conditions.
Sincerely,
The Trellus Elevate™ Team
A. Digital Notification and Alert System Overview
Trellus has designed a digital notification and alert system for your patients to be notified or alerted when they are reporting poor or worsening health trends. Additionally, they will be reminded of what labs require monitoring and when based on the medications they are receiving for their IBD and what health maintenance they are due for. Trellus educates patients on when and how to best communicate with their GI provider, to reduce the burden on you and your staff, and how to safely stay on their therapies and sustain tight control of their disease. Our automated disease monitoring and therapy notification system was designed specifically for GI providers based on best practice quality metrics.

- Red Flag notifications are triggered when your patient is reporting some poor health trends, including clinical and well-being outcomes, and or unplanned health care utilization which requires attention.
- Your patient will be advised to connect with you and your team to discuss this change in symptoms and next steps.
| Risk | Reason for Notification | |
| # of Bowel Movements | Patient is experiencing worsening of symptoms, with at least 4 bowel movements for 7 days in a row. | |
| Liquid Bowel Movements |
Patient is experiencing worsening of symptoms, reporting liquid stools for 7 days in a row. | |
| Blood in Bowel Movement |
Patient is experiencing worsening of symptoms, reporting blood in bowel movements for 7 days in a row. | |
| Urgency | Patient is experiencing worsening of symptoms, reporting urgency for 7 days in a row. | |
| Fecal Incontinence | Patient is experiencing worsening of symptoms, reporting leakage of stool, blood, or liquid before reaching the toilet at least 2 days in the past week. | |
| Abdominal Pain | Patient is experiencing worsening of symptoms, reporting moderate or severe abdominal pain for 7 days in a row. | |
| Urgent Care | Patient visited an urgent care center for their IBD. | |
| ER Visit | Patient visited the ER for their IBD. | |
| Hospitalization | Patient had an unplanned hospitalization for their IBD. | |
| GI Provider Contact | Patient has attempted to contact you but has not been able to reach you. | |
| CT Scan | Patient has received an unplanned CT scan. | |
| Prednisone Use | Patient has reported taking prednisone recently. | |
| Opioid Use | Patient has new report of taking opioid medication outside of their regular care plan. |

- Yellow Flag notifications are triggered when patient is reporting some poor health trends, including clinical and well-being outcomes, as well as key biomarkers of inflammation indicating there is rising risk of health care utilization.
- Your patient will be advised to connect with you and your team to discuss this change in symptoms and next steps.
| Trigger Field | Reason for Notification | |
| Bowel Movements | Patient had at least 4 bowel movements for 7 days in a row, with no worsening of symptoms in last 30 days | |
| Liquid Bowel Movements | Patient had liquid stools for 7 days in a row, with no worsening of symptoms in last 30 days | |
| Blood in Bowel Movements | Patient has blood in bowel movement 7 days in a row, with no worsening of symptoms in last 30 days | |
| Urgency | Patient is experiencing more frequent urgency 7 days in a row, with no worsening of symptoms in last 30 days | |
| Abdominal Pain | Patient had new mild pain at least 7 days in a row, with no worsening of symptoms in last 30 days | |
| Abdominal Pain | Patient had pain for at least 7 days in a row, more pain than usual, and has had pain at least 3 days per week in past 30 days | |
| General Physical Health |
Patient indicates worsening from previous week, now to “Fair” or “Poor” | |
| Average IBD- related Pain | Patient had pain > 4 or has had an increase of at least 3 levels since last report | |
| GI Provider Contact | Patient has reached out to you recently and had their concerns addressed. | |
| Fecal Calprotectin | Patient has a recent fecal calprotectin lab result greater than the upper limit of normal. | |
| C-Reactive Protein | Patient has a recent C-Reactive Protein lab result at least 2x the upper limit of normal. | |
| Hemoglobin | Patient has a recent Hemoglobin lab result of less than 11 gm/dL. | |
| Albumin | Patient has a recent Albumin lab result of less than 3.5 g/dL. |

These include labs, well being and symptom burden triggers that the Trellus Resilience Team will work with your patient to overcome and patients will be directed to their provider if there is a worsening in symptoms or other red or yellow flags are triggered.
| Notification | Trigger Field | Reason for Notification |
| Bloating | Patient reported feeling bloated for 7 days in a row. | |
| Fatigue | Patient reported two weeks of increased fatigue or very severe fatigue. | |
| Anxious, Depressed, or Irritable | Patient reported two weeks of being more bothered by emotional problems than usual, or often/always being bothered by emotional problems. | |
| Life Satisfaction | Patient reported disagreeing or strongly disagreeing with the statement: My life has been going well. | |
| Vitamin D 25-OH | Patient has a recent Vitamin D lab result of less than 30 ng/mL. |
B. Best Practices

TrellusElevate™ simplifies medication safety monitoring and preventative care/health maintenance with automated reminders that notify your patients when there are actions to be taken.
When adding new therapies or reviewing current medications, refer to the included Glossary of Medication-Related Monitoring and IBD Vaccine Reference Guide. We also provide these resources to your patients so they can increase their understanding of why these monitoring reminders are important and help to meet national standards.

TrellusElevate™ enables tight control disease monitoring based on clinical disease severity.
Biomarker Tracking
Remote patient monitoring is driven in part by patient’s lab results. Your patient may receive notifications to request a lab order from the provider if the patient is missing or overdue for the laboratory results in TrellusElevate™.
| Biomarker | High Clinical Severity | Lo w Clinical Severity |
| Fecal Ca lprotectin | At least every 4 months | At least every 6 months |
| C-Re active Protein | At least every 4 months | At least every 6 months |
GI Provider – Recommended Touch points
Based on disease severity we recommend the following clinical visit schedule
| High Clinical Severity | Low Clinical Severity |
| Every 4-6 months | Every 6 – 12 months |
C. Glossary of Medication Related Monitoring
| IBD Medications | Common Brands | Safety Checks |
| aminosalicylates – balsalazide, mesalamine |
Apriso™ Asacol® HD Colazal® Giazo™ Canasa® Delzicol™ Rowasa® Lialda™ Pentasa® |
Prior to Initiating Therapy While on Therapy |
| aminosalicylates – sulfasalazine |
Azulfidine® Azulfidine® EN- tabs | Prior to Initiating Therapy While on Therapy NOTE: More frequent lab monitoring may be necessary during first few months of therapy |
| biologics – TNF-α (adalimumab, infliximab, certolizumab pegol, golimumab) | Humira® Cyltezo™ Amjevita® Hyrimoz™ Cimzia® Simponi® Remicade® Renflexis® Avsola Inflectra™ IXIFI™ |
Prior to Initiating Therapy While on Therapy Patient Traveling Soon? |
| biologics – α4 integrin (natalizumab) |
Tysabri® | Prior to Initiating Therapy While on Therapy |
| biologics – IL-12/23 (ustekinumab) and IL-23 (risankizumab) | Stelara® Skyrizi ® |
Prior to Initiating Therapy While on Therapy Patient Traveling Soon? |
| biologics – α4β7 integrin (vedolizumab) | Entyvio® | Prior to Initiating Therapy While on Therapy |
|
corticosteroids – budesonide corticosteroids – prednisone |
Entocort® EC A-Methapred® Depo-Medrol® Medrol Dosepak® Solu-Medrol® Deltasone® Oraped® Prelone® Pediapred® |
✓ Daily Calcium (1200-1500 mg) and Vitamin D supplements (1000-2000 IU) * Generally, a bone density exam may be considered if patient has used oral corticosteroids lasting > 3 months, or if patient has used oral corticosteroids lasting 1 year within the past 2 years. *Women 65 or older should receive a bone density for preventative screening every 2 years regardless of steroid exposure. |
| immunosuppressant – cyclosporine |
Neoral® Sandimmune® Gengraf® | Prior to Initiating Therapy While on Therapy NOTE: More frequent lab monitoring may be necessary during first few months of therapy |
| immunosuppressant – methotrexate |
Rheumatrex® MTX® Mexate® Trexall® |
Prior to Initiating Therapy While on Therapy NOTE: More frequent lab monitoring may be necessary during first few months of therapy Patient Considering Pregnancy? |
| immunosuppressant – tacrolimus |
Prograf® Astagraf XL® Envarsus® XR Hecoria® |
Prior to Initiating Therapy While on Therapy NOTE: More frequent lab monitoring may be necessary during first few months of therapy |
| immunosuppressant – thiopurine, azathioprine, 6- Mercaptopurine (6-MP) | Imuran® Azasan® Purinethol® Purixan | Prior to Initiating Therapy While on Therapy NOTE: More frequent lab monitoring may be necessary during first few months of therapy |
| small molecules – JAK- inhibitor (tofacitinib, upadacitinib) | Xeljanz® Rinvoq® |
Prior to Initiating Therapy While on Therapy NOTE: More frequent lab monitoring may be necessary during first few months of therapy Patient Traveling Soon? Patient Considering Pregnancy? |
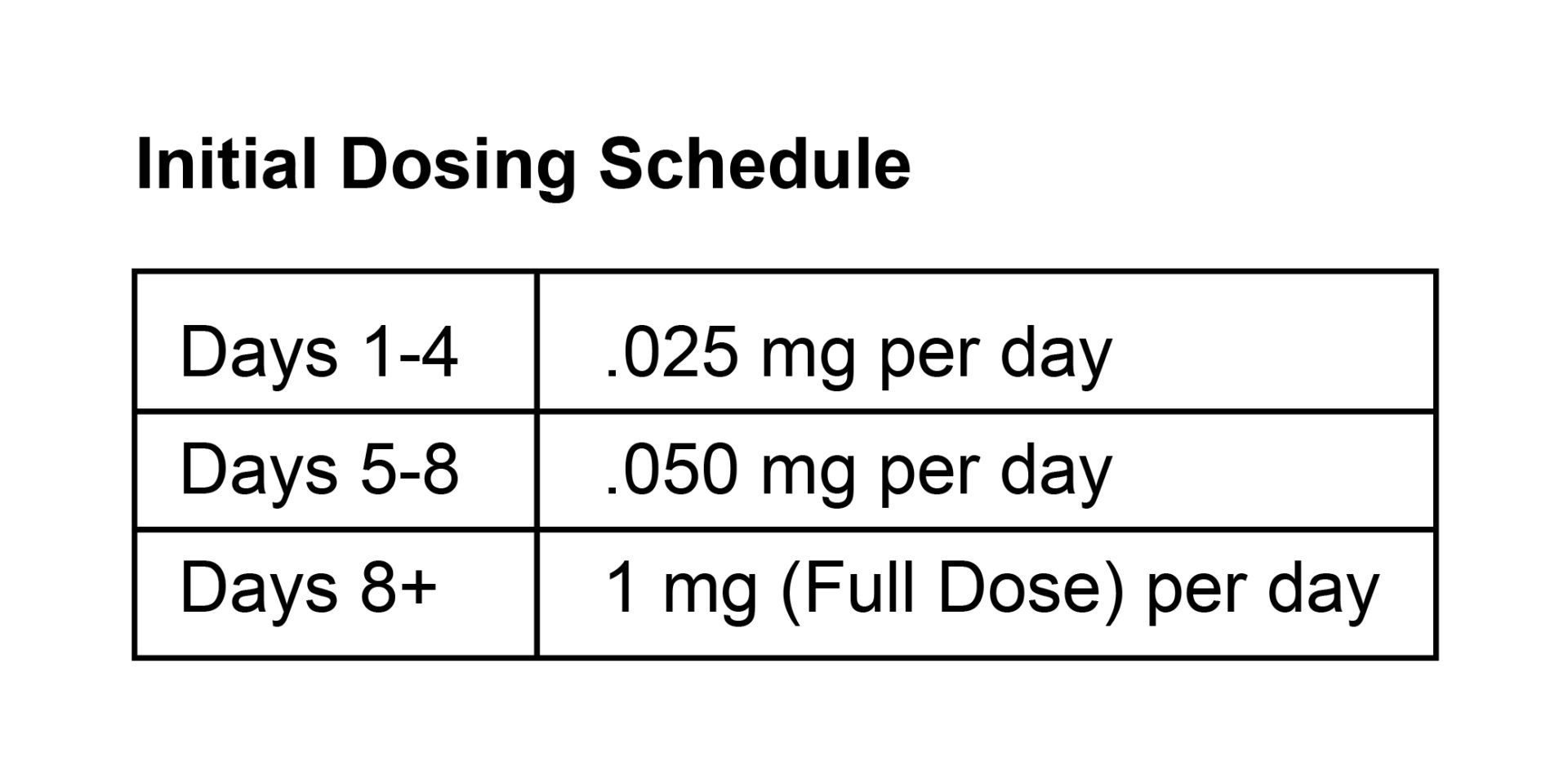
| small molecules – S1P Receptor Molecules (ozanimod) | Zeposia® | Prior to Initiating Therapy While on Therapy ✓ Routine bloodwork such as complete blood count, renal function, liver function every 3-6 months Initial Dosing Schedule NOTE: More frequent lab monitoring may be necessary during first few months of therapy Patient Considering Pregnancy? |
D. IBD Vaccine Reference Guide
| Vaccine | Vaccine Type Live vs. Non-Live | Information & Tips |
| COVID-19 | Non-live | Stay Up to Date with Covid-19 Vaccines at https://www.cdc.gov/ coronavirus/2019-ncov/vaccines/stay-up-to-date.html |
| “Chicken Pox” Varicella (VAR) |
Live This vaccine should not be administered when taking certain IBD medications, such as biologics or immunosuppress ants including corticosteroids |
The varicella vaccine is the best way to protect patients from getting the chicken pox. People 13 years of age and older who have never had chickenpox or received the chickenpox vaccine should get two doses, at least 28 days apart. The chickenpox vaccine was added to the childhood immunization schedule in 1995, so most people born prior to 1995 had a higher likelihood of getting chickenpox and getting immunity that way. Note that even if your patient had chickenpox in the past, their immunity may have weakened over time and the titers can be checked. If low titers patient may be recommended to receive 2 doses of the chickenpox vaccine. Patients on low-dose immunosuppression may consider the chickenpox vaccine (e.g., ≤ 20 mg/day prednisone,≤0.4 mg/kg/week MTX, ≤ 1.5 mg/kg/day 6-MP, ≤ |
| “Flu” Influenza inactivated (IIV) |
Non-live when given as an injection Live when given intranasally |
The flu shot (non-live) and not the nasal mist (live vaccine) is recommended If possible, members of the household should also opt for the flu shot and avoid the nasal mist. |
| Hepatitis A (HepA) | Non-live | The CDC recommends the HepA vaccine for special situations such as prior to traveling to specific countries. |
| Hepatitis B (HepB) | Non-live | Your patient can protect against Hepatitis B infections/reinfections by receiving the Hepatitis B vaccine series, especially if starting or already receiving any Anti-TNF (Remicade® and infliximab biosimilars, Humira®, Cimzia®, Simponi®). |
| Herpes Zoster recombinant (RZV) | Non-live | Getting the Shingrix (Herpes Zoster) vaccine is the best way to protect against getting shingles, especially when patients are on certain IBD therapies (Xeljanz®, Rinvoq®, Zeposia®, Azathioprine, 6-MP) or if above age 50 or above the age of 18 and are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy. |
| Human papillomavirus (HPV) | Non-live | The HPV vaccine can protect against some HPV types that commonly cause cervical cancer and genital warts. The CDC recommends the HPV vaccine for males and females between the ages of 9 and 26 and some adults aged 27 to 45 years. |
| Measles, Mumps, and Rubella (MMR) |
Live This vaccine should not be administered when taking certain IBD medications, such as biologics or immunosuppress ants including corticosteroids |
The MMR vaccine protects patients against Measles, Mumps, and Patients on low-dose immunosuppression may consider the chickenpox vaccine (e.g., ≤ 20 mg/day prednisone,≤0.4 mg/kg/week MTX, ≤ 1.5 mg/kg/day 6-MP, ≤ 3 mg/kg/day azathioprine.) |
| Meningococcal A, C, W, Y (MenACWY) |
Non-live |
Meningococcal A, C, W, Y (MenACWY) is typically given at 11-12 years, and 16 years of age. This vaccine is recommended for first-year college students who live in residential housing and military recruits. |
| Meningococcal B (MenB) | Non-live | Meningococcal group B (MenB) recommended for those 16-23 years old |
| Pneumococcal conjugate (PCV13) (PCV15) (PCV20) | Non-live |
PCV13 (Prevnar 13®) PCV15 (Prevnar15®), PCV20 (Prevnar20® )is a routine vaccination for those with chronic medical conditions, like IBD, that can protect patients against pneumonia. Prevnar 7 ® was a routine vaccination for infants up until February 2010, when the other Prevnar vaccines replaced it. Studies show that patients with IBD, regardless of age, would benefit from this one-time vaccination. PPSV23 can be given at least 8 weeks from PCV13 and PCV15. PPSV23 is not recommended if the patient received PCV20. |
| Pneumococcal polysaccharide (PPSV23) | Non-live | PPSV23 (Pneumovax® 23) is a routine vaccination for those with chronic medical conditions, like IBD, that can protect patients against pneumonia. This should be administered at least 8 weeks after PCV13 or PCV15. They do not need PPSV23 if they received PCV20 |